Mass Molarity Calculator
iOS Universel / Utilitaires
Mass Molarity Calculator is a useful tool that allows you to calculate the mass of a compound required to prepare a solution of known volume and concentration.
An example of a molarity calculation using the Mass Molarity Calculator:
What is the mass of the compound required to make a 10 mM stock solution in 10 ml of water, given that the molecular weight of the compound is 197.13 g/mol?
How to calculate mass:
Mass (m) is the amount of matter present in a substance. The value is constant and, unlike weight, is not affected by gravity.
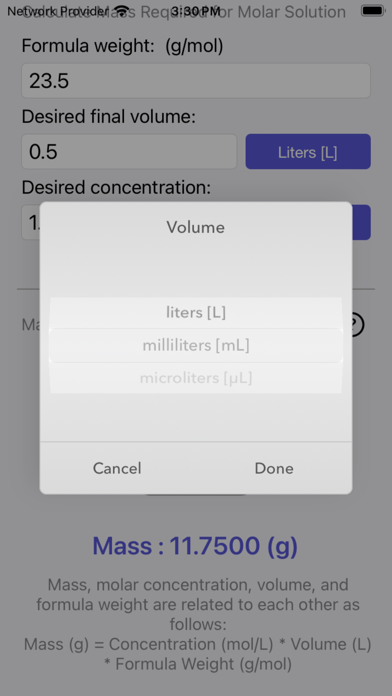
Mass, molar concentration, volume, and formula weight are related to each other. The Mass Molarity Calculator is based on the following equation:
Mass (g) = Concentration (mol/L) * Volume (L) * Formula Weight (g/mol)
Formula weight (F.W.) is the sum of the atomic weights of all atoms in a given empirical formula. For example, Sodium chloride (NaCl) has one atom of sodium (Na) and one atom of chlorine (Cl). The atomic weight of sodium is 22.99 g/mol, and chlorine is 35.45 g/mol. Therefore, the formula weight of NaCl is 58.44 g/mol (22.99 g/mol + 35.45 g/mol).
The included chemical elements are listed by atomic mass for fast reference. The table includes Atomic number, Atomic Mass, Symbol, and Chemical Element name.
Molar concentration is the amount of a solute present in one unit of a solution. Its units are mol/L, mol/dm3, or mol/m3. “Molar concentration” is also known as “molarity” and can be denoted by the unit M, molar. If we want to prepare 1 L of 0.5 M sodium chloride solution, then as per the formula, we require 29.22 g of sodium chloride (0.5 mol/L * 1L * 58.44 g/mol = 29.22 g).
The Mass Molarity Calculator tool calculates the mass of the compound required to achieve a specific molar concentration and volume.
Thanks for your support, and do visit nitrio.com for more apps for your iOS devices.
Quoi de neuf dans la dernière version ?
- Updated for the newest devices.
- Minor bugs fixed.